GXPTrace™
End-to-End Pharmaceutical Track & Trace Solutions
In today’s highly regulated pharmaceutical environment, securing the supply chain is no longer optional — it is essential.
With the World Health Organization estimating that approximately 10.5% of medicines worldwide are substandard or falsified, pharmaceutical companies must adopt robust, technology-driven safeguards. GXPTrace™ by GXPWAY delivers advanced track and trace solutions that create a secure, transparent, and fully compliant supply chain — from manufacturing to patient dispensing.
What is Pharmaceutical Track & Trace?
Track and trace systems ensure the integrity, authenticity, and traceability of medicinal products across the entire product lifecycle.
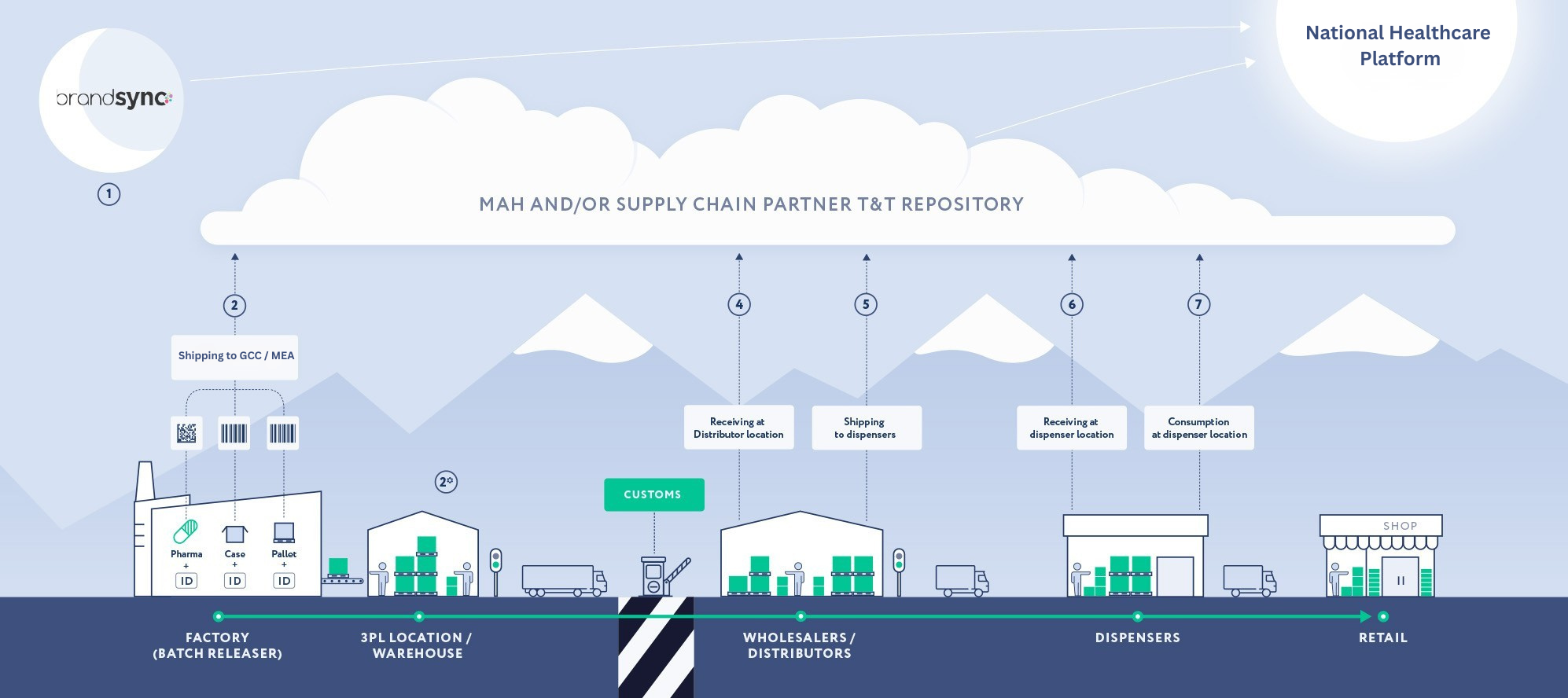
As pharmaceutical supply chains expand globally, every transfer point — from manufacturer to distributor, wholesaler, pharmacy, and hospital — represents a potential vulnerability. GXPTrace™ eliminates blind spots by creating an unbroken digital chain of custody, ensuring:
Protection against counterfeit medicines
Real-time product visibility
Regulatory compliance across multiple jurisdictions
Faster recall management
Enhanced patient safety
Beyond regulatory compliance, track and trace technology strengthens operational performance across the entire pharmaceutical ecosystem.
Benefits Across the Pharmaceutical Ecosystem
For Manufacturers
Product serialization and aggregation
Enhanced brand protection
Improved inventory accuracy
Reduced recall impact
Global regulatory compliance
For Distributors & Wholesalers
Automated shipment verification
Reduced liability exposure
Real-time supply chain visibility
Efficient recall processing
For Pharmacies & Healthcare Providers
Instant authentication verification
Automated expiration tracking
Improved stock management
Reduced dispensing errors
For Patients
Protection against counterfeit or falsified medicines
Increased trust in medication safety
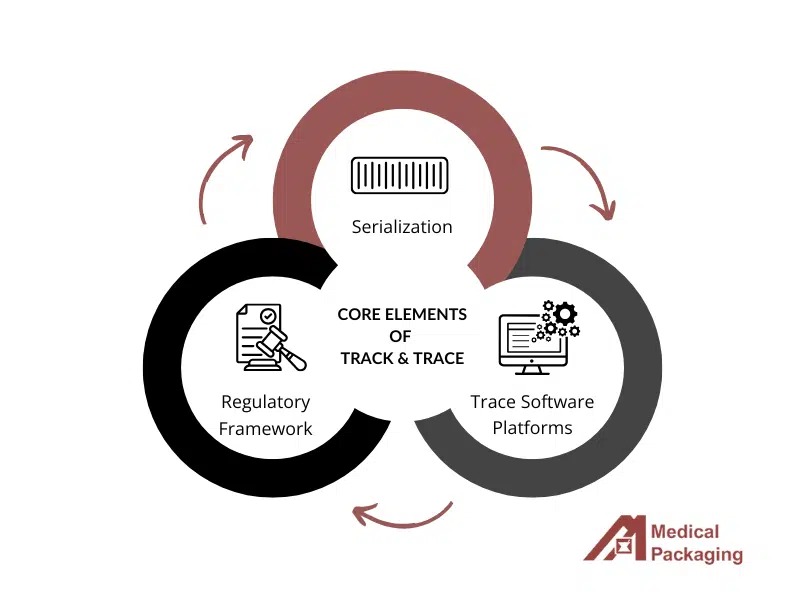
How GXPTrace™ Works
GXPTrace™ integrates three core components to ensure seamless compliance and supply chain transparency:
1. Pharmaceutical Serialization
Each product pack is assigned a unique identifier, including:
Product code
Unique serial number
Batch/lot number
Expiry date
High-precision packaging equipment and advanced barcode labeling systems apply secure DataMatrix codes, ensuring each pack is individually traceable.
2. Real-Time Traceability Platform
GXPTrace™ software monitors product movement across the supply chain.
Each scan generates a secure digital transaction record
Data updates in real time
Pharmacists and hospitals can instantly verify product authenticity
Automated alerts notify stakeholders of recalls or status changes
This creates a secure digital audit trail accessible across authorized stakeholders.
3. Global Regulatory Compliance Framework
Track and trace systems are driven by international regulatory requirements, including:
Falsified Medicines Directive (EU FMD)
Drug Supply Chain Security Act (U.S. DSCSA)
GXPTrace™ is designed to support multi-market compliance, enabling pharmaceutical companies to operate efficiently across the UK, EU, U.S., and other global markets while maintaining continuous regulatory alignment.
Applications in Pharmacies, Hospitals & Healthcare Facilities
Pharmacies
Instant verification upon product receipt
Automated recall and expiration alerts
Simplified regulatory audits
Improved inventory control
Hospitals
Full visibility of medication movement within the facility
Optimized inventory across satellite pharmacies
Reduced emergency orders
Integration with electronic health record (EHR) systems
When integrated with clinical systems, barcode scanning before administration ensures the right patient receives the right medication — significantly reducing medication errors.
Measurable Impact
Healthcare facilities implementing track and trace systems report:
Faster recall processing
Reduced stock discrepancies
Improved reimbursement documentation
Decreased medication waste
Challenges in Pharmaceutical Track & Trace — and How GXPTrace™ Addresses Them
Implementing serialization and traceability systems presents several challenges:
Fragmented global regulatory frameworks
Evolving compliance requirements
Complex system integration
Increasingly sophisticated counterfeit methods
GXPTrace™ provides a flexible, scalable architecture capable of adapting to evolving regulations while maintaining operational efficiency. Continuous security updates and advanced authentication features help protect against emerging counterfeiting techniques.
Why Choose GXPWAY GXPTrace™
Designed for GMP & GDP regulated environments
Multi-market regulatory compliance capability
Seamless ERP and MES integration
Scalable for manufacturers, distributors, and healthcare providers
Supports inspection readiness and audit traceability
GXPTrace™ transforms compliance from a regulatory obligation into a strategic advantage — delivering transparency, operational efficiency, and enhanced patient safety across the pharmaceutical supply chain.


